WHAT IS TELARA PHARMA
The scientific team of TELARA has spent years studying toxic and non-toxic aggressions suffered by the kidneys with the aim of preventing and treating AKI. The company arises out of the pioneering work on nephroprotection carried out with cilastatin by the team of the KOL Dr. Alberto Tejedor to cover a medical need that is not currently addressed.
WHAT WE DO WE DO BEST
About us


why we do what we do? Telara Pharma
The scientific team of TELARA has spent years studying toxic and non-toxic aggressions suffered by the kidneys with the aim of preventing and treating AKI.
The company arises out of the pioneering work on nephroprotection carried out with cilastatin by the team of the KOL Dr. Alberto Tejedor to cover a medical need that is not currently addressed.
Acute kidney Injury (AKI) is a syndrome that results in a sudden decrease in kidney function and currently is one of the most serious and common health problems in the world associated with severe morbidity and mortality as well as the development of chronic kidney disease (CKD).
Every year, it affects around 13.3 million people, causing 1,7 million deaths. It therefore represents a true epidemic in today’s nephrology where 1 in 5 adults and 1 in 3 children worldwide experience AKI during a hospital episode of care with a mortality rate of 50-80%.
Cilastatin is a specific competitive blocker of renal dehydropeptidase I (DHP-I) enzyme which is located on brush-border cholesterol rafts of renal tubular cells.
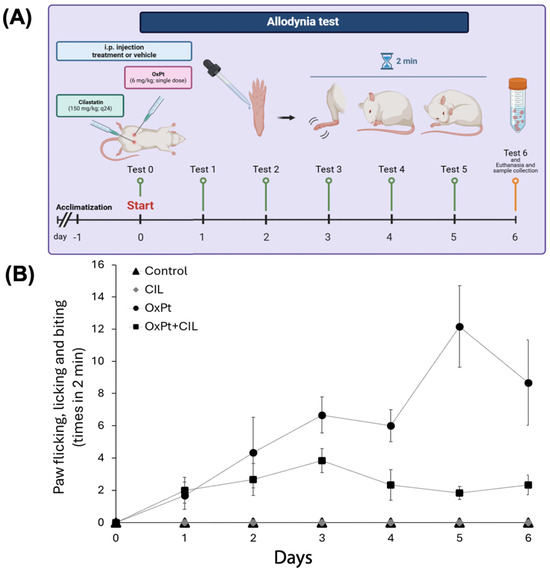
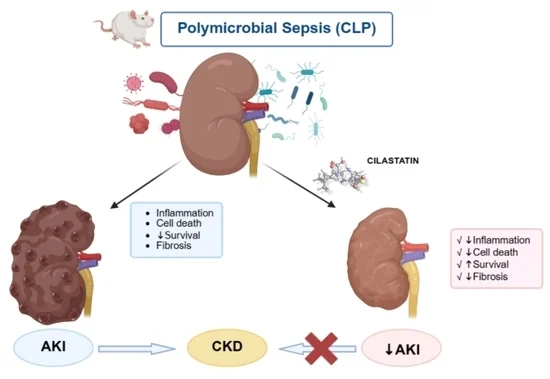
Our preclinical in vitro and in vivo studies have demonstrated that cilastatin protects the kidney against tubular cell injury and acute kidney injury (AKI) caused by nephrotoxic drugs such as cisplatin, cyclosporine and gentamicin, and other non-toxic conditions such as sepsis.
SCIENTIFIC DATA
Cilastatin is an specific inhibitor of Dipeptidase 1 (DPEP1).
Cilastatin blocks the apoptosis process.
Cilastatin avoids the internalization via cholesterol rafts of the Fas/FasL complex preventing the extrinsic pathway of apoptosis.
Cilastatin is also effective in a non-toxic AKI such as that caused by sepsis.
Meet the TEAM
Check our
PIPELINE
| Program | Indication | Pre-clinical | Phase I | Phase II | Phase III | Partners | |
| TLP-01 |
ACUTE KIDNEY INJURY caused by Drugs
|
Licensed to: |
|||||
| TLP-02 |
ACUTE KIDNEY INJURY caused by Sepsis
|
Licensed to: |
|||||
| TLP-03 |
GLAUCOMA
|
wholly-owned | |||||
| TLP-04 |
Undisclosed
|
wholly-owned | |||||
Need to contact us?
See the latest news